Why care about nitrogen?



Nitrogen ranks fourth as the most common chemical element in living tissues. Before human contribution to the nitrogen content in the atmosphere, nitrogen was a major limiting factor controlling the functioning of ecosystems (Marris, 2008)! Despite 78% of earth’s atmosphere being nitrogen, most plants and animals must wait for the nitrogen to be ‘fixed’. This occurs through its bonding with hydrogen or oxygen to form ammonium and nitrate (Fields, 2004).
The current measurement of fixed nitrogen occurs in tetragrams (Tg), which is equal to a million metric tons of nitrogen. It is estimated that the rate of natural nitrogen fixation on land is 140Tg of N per year (Vitousek et al., 1997). Surprisingly, human activities have resulted in an extra artificial nitrogen fixation of 218Tg of N per year (Vitousek et al., 1997)!
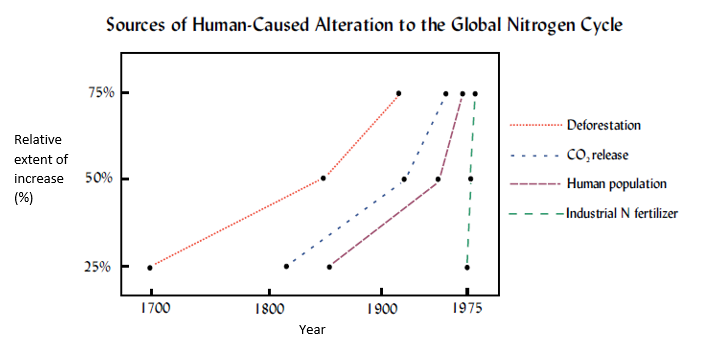
More worryingly is that by 2050, if predicted population trends are accurate then artificial N fixation will reach four times that of the natural production rate(Tilman & Lehman, 2001)!
Why is this important?
One of the biggest problems nitrogen poses to aquatic ecosystems, is its ability to form nitric acid through complex chemical reactions! This acid results in increasing the concentration of H+ in freshwater environments. This results in the PH of the water decreasing. A decreasing PH can result in an increase in the concentration of trace metals (Lee & Saunders, 2003). This occurs due to decreased metal sedimentation. The settling of dissolved aluminium reduces phosphate availability and therefore affects the phosphate cycle (Camargo & Alonso, 2006).
This is devastating! Phosphates are involved in the formation of ATP during respiration, and this ATP is essential for the normal functioning of an organisms metabolism, and without proper functionality, death will occur. A PH below 6 seems to be the threshold for significant damage. In fish, arrested development of embryos can occur, resulting in skeletal deformities (Camargo & Alonso, 2006).
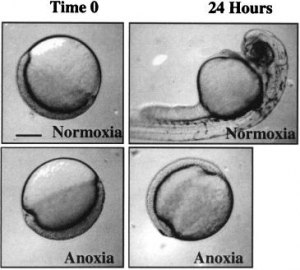
High nitrogen levels can disrupt ionic regulation, which results in molluscs, insects, fish and amphibians suffering from a deficit in calcium. This causes issues with bone development and shell maturation. In terms of the food chain, the reduced PH of aquatic environments causes a depression of net photosynthesis in planktonic and attached algae (Eisler, 2012) . This is due to the increased growth of algae which can ‘cloud’ the water preventing light penetration, as well as through disproportional oxygen consumption.
Moreover, the decline in dissolved oxygen can promote the production of hydrogen sulphide by anaerobic bacteria. Hydrogen sulphide can further reduce the availability of oxygen, due to it accumulating at the waters surface (Smith & Oseid, 1974)! This is a huge problem as dissolved oxygen is essential to the respiration of aquatic organisms, and without it, death is inevitable!
Are humans really responsible?
In short, yes, we are to blame!
If the current trend continues, not only will the current effects be amplified, resulting in a greater number of deaths, but the cost of aquatic organisms as a source of food will sky-rocket!
References
Camargo J, Alonso Á. (2006). Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environment International, 32(6), pp.831-849.
Eisler R. Oceanic acidification. (2012). 1st ed. Boca Raton: CRC Press
Fields S. (2004). Global Nitrogen: Cycling out of Control. Environmental Health Perspectives, 112(10), pp.556-563.
Lee M, Saunders J. (2003). Effects of pH on Metals Precipitation and Sorption: Field Bioremediation and Geochemical Modeling Approaches. Vadose Zone Journal, 2(2):, pp.77-185.
Marris, E. (2008). Nitrogen pollution stomps on biodiversity. Nature, 1, pp.1-3.
Smith L, Oseid D. (1974). Effect of Hydrogen Sulfide on Development and Survival of Eight Freshwater Fish Species. The Early Life History of Fish, 1, pp.417-430.
Tilman D, Lehman C. (2001). Human-caused environmental change: Impacts on plant diversity and evolution. Proceedings of the National Academy of Sciences, 98(10), pp.5433-5440.
Vitousek, P., Aber, J., Howarth, R., Likens, G., Matson, P., Schindler, D., Schlesinger, W. and Tilman, D. (1997). Technical Report: Human Alteration of the Global Nitrogen Cycle: Sources and Consequences. Ecological Applications, 7(3), pp.737-750
[word count: 487]
Recent Comments