As we enjoy a varied diet of carbohydrates, proteins and fats, for plants it is the gas carbon dioxide (CO2), water and sunlight (figure. 1).

It is not just CO2 use which is made more efficient at elevated CO2 levels, water efficiency is greater as less water is lost from leaf pores; stomata. FACE (Free-Air CO2 Enrichment) experiments with soybean show that leaf pore conductance is not adapted to elevated CO2 but rather maintain decreased conductance. Furthermore, this increase in water efficiency is consistent between the leaf and canopy levels (Leakey et al, 2009).
It was also thought that higher CO2 levels lead to increased efficiency of nitrogen, a mineral required for growth, as plants grown at these levels do not have as much nitrogen present. These high CO2 grown plants also have a greater biological mass than those grown at normal CO2 conditions. However these CO2 levels where not found to affect levels of biological mass attained over plant lifetime which indicates that an accelerated period of growth that used up nitrogen reserves (Coleman et al, 1993). Increased CO2 levels are thought to contribute to increased uptake of nitrogen by plant roots rather than increased plant efficiency regarding nitrogen. Further FACE experiments at three separate forest locations showed that increased biological mass corresponded to increased nitrogen uptake from the soil. However this is limited to areas where nitrogen soil supply exceeds demand and is therefore unlikely to be seen in all plants worldwide (Finzi et al, 2007).
These FACE experiments are advantageous as they allow CO2 to be applied to a specific area of a wide range of ecosystems from desert to tropical forest. Trees as tall as 25m can be used in these experimental plots which can be as large as 30m in dimeter (Norby and Zak, 2011).
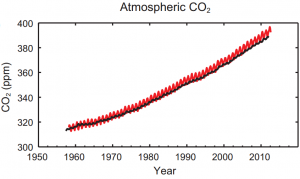
Scientists have been documenting rising atmospheric CO2, which is associated with planetary warming, for almost 70 years now, since 1950 (figure. 2). It is widely accepted that this change in CO2 has arisen from human industrialisation. While it seems that plants can positively cope with this change this conclusion must not be taken at face value and further studies must be undertaken.
- Coleman, J.S., McConnaughay, K. D. M and Bazzaz, F. A. (1993). Elevated CO2 and Plant Nitrogen-Use: is reduced Tissue Nitrogen Concentration Size Dependent?. Oecologia. 93, 195-200.
- Drake, B. G., Gonzalez-Meler, M. A and Long, S. P. (1997). More Efficient Plants: a Consequence of Rising Atmospheric CO2. Ann. Rev. Plant. Physiol. 48, 609-639.
- Finzi, A. C., Norby, R. J., Calfapietra, C., Gallet-Budynek, A., Gielen, B., Holmes, W. E., Hoosbeek, M. R., Iversen, C. M, Jackson, R. B., Kubiske, M. E, Ledford, J., Liberloo, M., Oren, R., Polle, A., Pritchard, S., Zak, D. R., Schlesinger, W. H and Ceulemans, R. (2007). Increased in Nitrogen Uptake rather than Nitrogen-Use Efficiency support higher rates of Temperate Productivity under Elevated CO2. PNAS. 104 (35), 14014-14019.
- IPCC, 2013: Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Leakey, A. D. B., Ainsworth, E. A., Bernacchi, C. J., Rogers, A., Long, S.P and Ort, D. R. (2009). Elevated CO2 Effects on Plant Carbon, Nitrogen and Water Relations: six important lessions from FACE. Journal of Experimental Botany. 60 (10), 2859-2876.
- Lind, J. © Photo of Pedunculate Oak Tree. Available: http://www.arkive.org/pedunculate-oak/quercus-robur/image-A20783.html. Last accessed 20th March 2017.
- Norby, R. J and Zak, D. R. (2011). Ecological Lessons from Free-Air CO2 Enrichment (FACE) Experiments. Annual Review of Ecology, Evolution and Systematics. 42. 181-203.
[480 words]



Recent Comments