
I’m sure you’ve all seen enough TV adverts showing polar bears floating on small patches of ice by now to just roll your eyes and change channel. I’m also sure you’ve thought to yourself “Why is it always polar bears?” and “Why should I care?”. I’d like to explain why polar bears and, more broadly, arctic ice are so important and why, instead of averting our eyes, we should be paying more attention than ever.
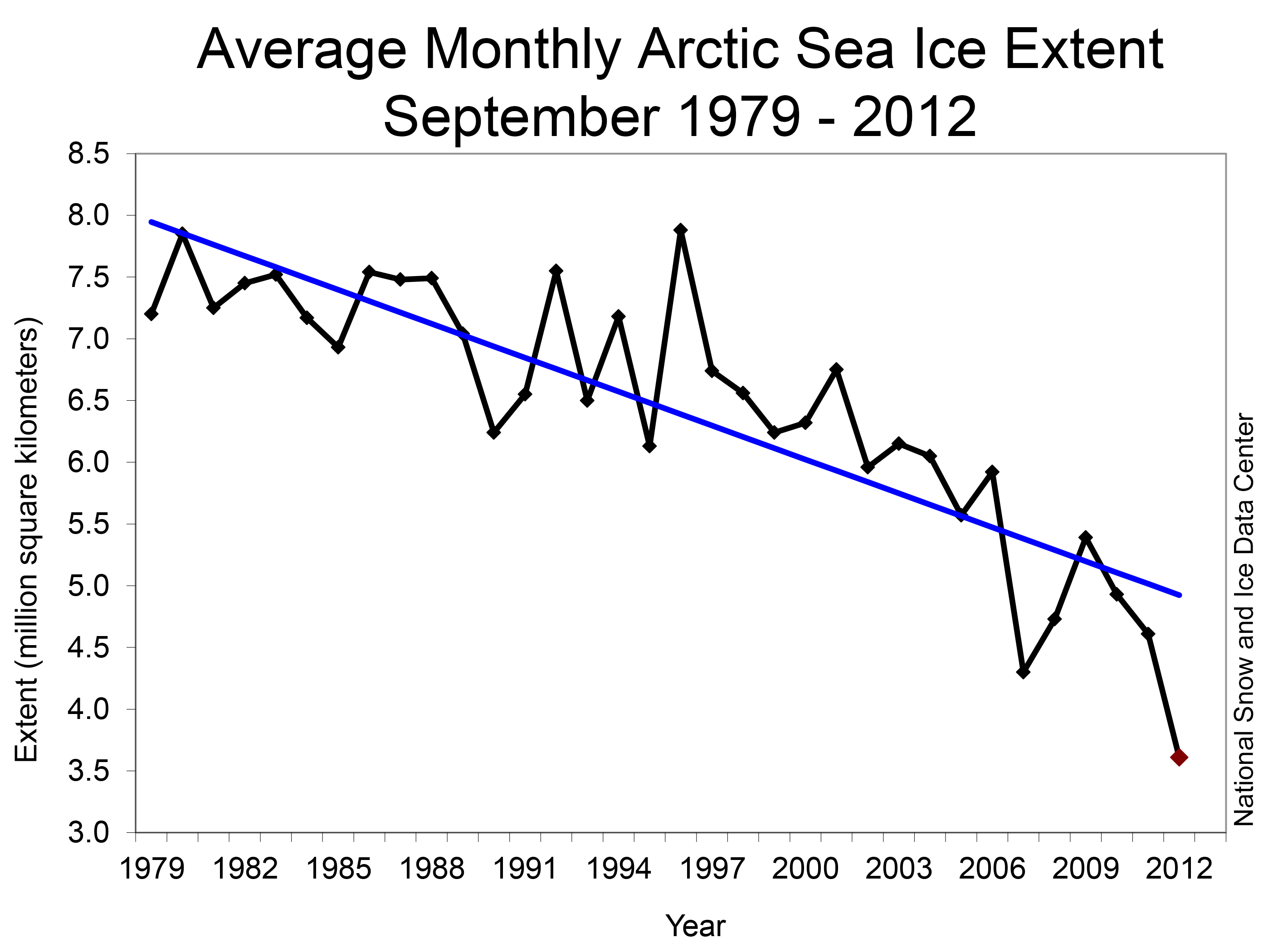
Most scientists agree that polar bears are in serious trouble. With their habitat and hunting ground of choice, arctic sea ice, disappearing at a rate of 13 percent per year (Nsidc.org, 2017) polar bears are getting thinner (Obbard et al., 2016) and those cute baby polar bears are bearing the brunt of it (Pagano et al., 2012). Polar bears use the ice as platforms to catch their main source of food, ringed and harp seals, with a surprising amount of stealth and guile for 2.5m long, 700 kilo apex predators.

With longer swims required to reach the ice each year, fewer polar bear cubs can make the swim (Pagano et al., 2012 and Hassol, 2004). No more cubs mean no more polar bears. No more polar bears would have enormous knock-on effects on the artic ecosystem. For example, if harp seal numbers were to increase due to lack of predation, numbers of Northern shrimp would drop significantly (Hassol, 2004). Northern shrimp have no fewer than 16 predators and so act as a lynchpin for a significant portion of the food web (Parsons, 2005).
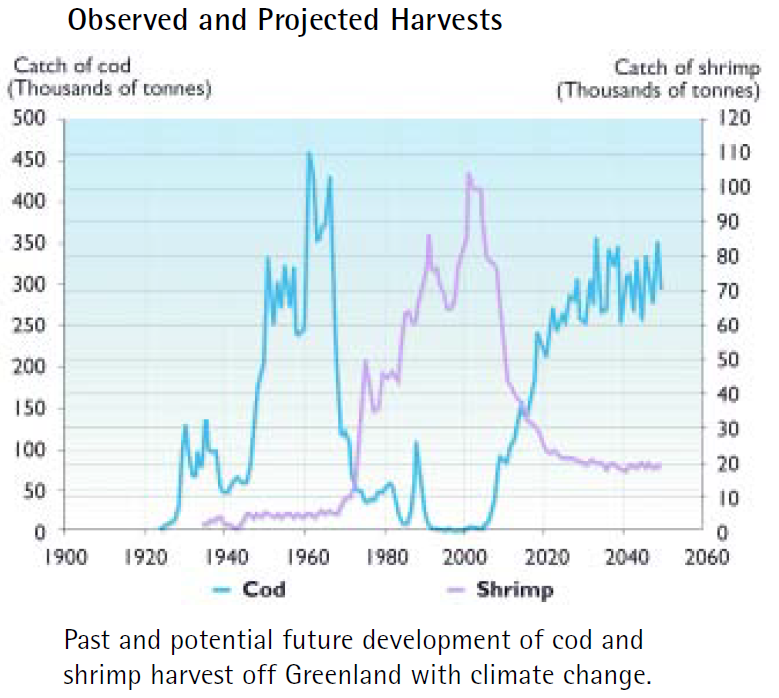
Why does this matter then? More than anything, the plight of the polar bear represents our success or failure in safeguarding our planet. Breaking apart the food web of the arctic doesn’t just affect the ecosystem there; If we continue the path we are currently on, then we risk damaging the ecosystem to the point where we lose out on 7 million tons of fish every year (Hassol, 2004 and EPA, 2017).
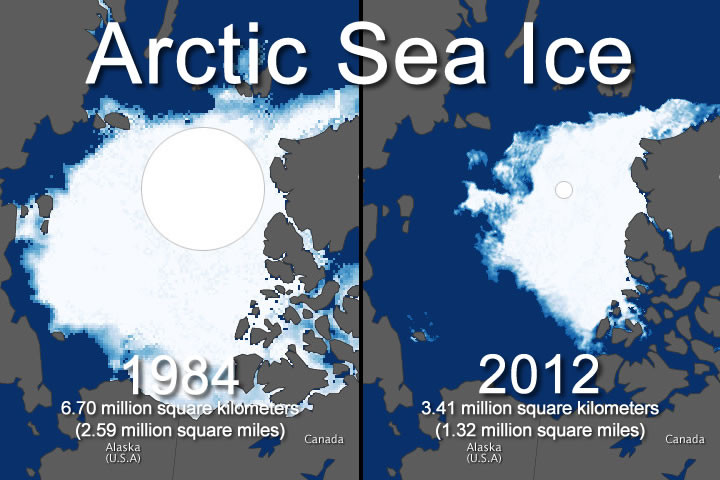
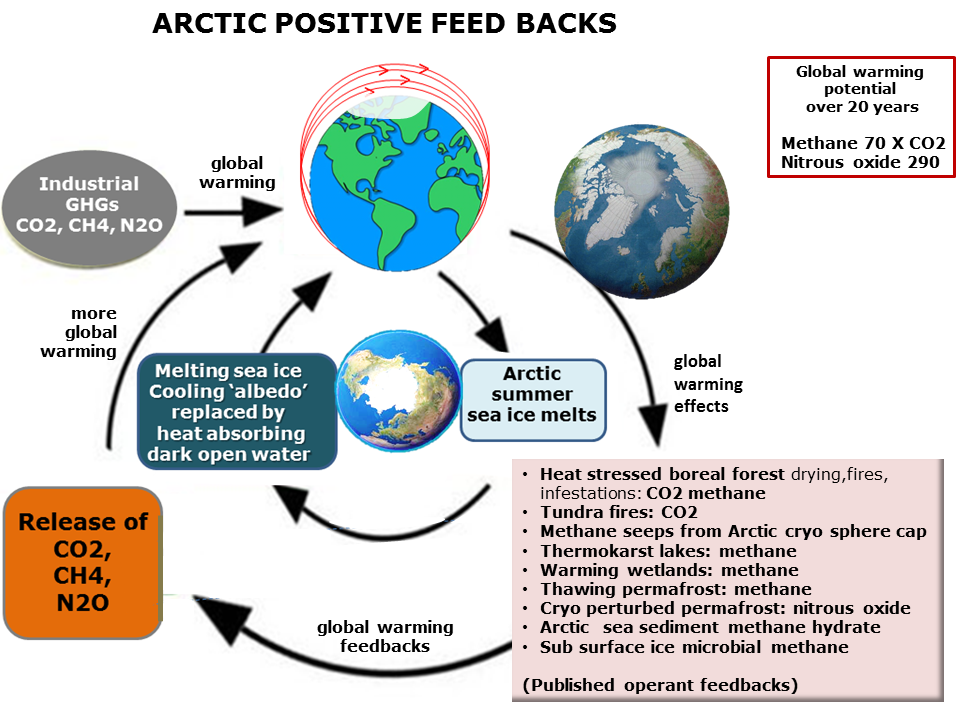
Damage to the arctic ecosystem doesn’t stop at polar bears and fish. The effects of arctic ice reduction will be felt all over the globe from rising sea levels to the loss of cold-adapted species, such as the humble lemming. With enough warming, those cold-adapted species will struggle to survive while other invasive species will suddenly have massive stretches of land made available to them (Ware, 2013). The most serious problem, however, is that we may have set off a chain of events that is running far out of our control.
It isn’t all doom and gloom of course, its predicted that we’ll see more trans-arctic marine transport and (unfortunately) better access to arctic oil deposits which will help off-set the damage (Hassol, 2004 and Block, 2016). The question we need to ask ourselves, however, is “Will it be worth it?

Word count: 485
References:
- Nsidc.org. (2017). Arctic Sea Ice News and Analysis | Sea ice data updated daily with one-day lag. [online] Available at: http://nsidc.org/arcticseaicenews/ [Accessed 20 Mar. 2017].
- Obbard, M., Cattet, M., Howe, E., Middel, K., Newton, E., Kolenosky, G., Abraham, K. and Greenwood, C. (2016). Trends in body condition in polar bears ( Ursus maritimus ) from the Southern Hudson Bay subpopulation in relation to changes in sea ice. Arctic Science, 2(1), pp.15-32.
- Pagano, A., Durner, G., Amstrup, S., Simac, K. and York, G. (2012). Long-distance swimming by polar bears ( Ursus maritimus ) of the southern Beaufort Sea during years of extensive open water. Canadian Journal of Zoology, 90(5), pp.663-676.
- Hassol, S. (2004). Impacts of a warming Arctic. 1st ed. [ebook] Cambridge: Cambridge University Press. Available at: http://www.cambridge.org/gb/academic/subjects/earth-and-environmental-science/climatology-and-climate-change/impacts-warming-arctic-arctic-climate-impact-assessment?format=PB&isbn=9780521617789 [Accessed 20 Mar. 2017].
- Parsons, D. (2005). Predators of northern shrimp,Pandalus borealis(Pandalidae), throughout the North Atlantic. Marine Biology Research, 1(1), pp.48-58.
- gov. (2017). Climate Impacts on Ecosystems | Climate Change Impacts | US EPA. [online] Available at: https://www.epa.gov/climate-impacts/climate-impacts-ecosystems [Accessed 20 Mar. 2017].
- Ware, C. (2013). Arctic at risk from invasive species. [Blog] The Ecologist. Available at: http://www.theecologist.org/News/news_analysis/2173097/arctic_at_risk_from_invasive_species.html [Accessed 20 Mar. 2017].
- Block, B. (2016). Arctic Melting May Lead To Expanded Oil Drilling | Worldwatch Institute. [online] Worldwatch.org. Available at: http://www.worldwatch.org/node/5664 [Accessed 20 Mar. 2017].
Figures:
Figure 1 – Polar bear on ice – Available from: http://www.endangeredpolarbear.com/uploads/1/4/2/4/14243313/2894929.png
Figure 2 – Polar bear hunting seal – Available from: https://i.ytimg.com/vi/MC26JK9nk-8/maxresdefault.jpg
Figure 3 – Arctic ice loss from 1984-2012 – Available from: https://intellihub.com/wp-content/uploads/2015/01/1348775537_2624_composite.jpg
Figure 4A – Past and predicted fishing – Available from Reference number 3.
Figure 4B – Arctic sea ice loss graph – Available from: http://nsidc.org/images/arcticseaicenews/20101004_Figure3.png
Figure 5 – Arctic warming positive feedback loop – Available from: http://www.climateemergencyinstitute.com/uploads/arctic_fb1.png
Figure 6 – The Arctic is not for sale – Available from: http://www.k-zap.org/wp-content/uploads/2015/12/arctic-drilling.jpg



















Recent Comments