 Such tragic pictures were taken in China telling stories of the low-income people who live on fisheries lost their fishes due to the algae bloom. However, this problem does not only present in China: according to reports, during 1972 to 1999 US commercial fisheries lost over 18 million dollars every year due to the poor water quality (National Science Foundation, 2000).
Such tragic pictures were taken in China telling stories of the low-income people who live on fisheries lost their fishes due to the algae bloom. However, this problem does not only present in China: according to reports, during 1972 to 1999 US commercial fisheries lost over 18 million dollars every year due to the poor water quality (National Science Foundation, 2000).

How is it developed?
Under warm and excessive nutrient conditions (e.g., introduction of nitrogen and phosphorus), algae in the lake starts to grow rapidly. In most healthy lakes, all depths are well oxygenated and the species in lakes are diverse. The excessive nutrient loads leads to the dominance of algae to the lakes, in other word, algae bloom. Massive algae bloom in the surface results in water turbidity increasing therefore the sunlight is blocked from underwater plants. Additionally, algae in the lakes has a short lifespan and depletes the oxygen in water causing a death zone along the water column (hypoxia); some algae can release toxins which are deadly to fish (Hallegraeff, 1993). In this stage the amount of fishes along with aquatic plants decreases rapidly. The healthy, well-oxygenated and clear lake becomes turbid, unsightly with few species alive and a disgusting smell.
What happens to ecosystem within the lake?
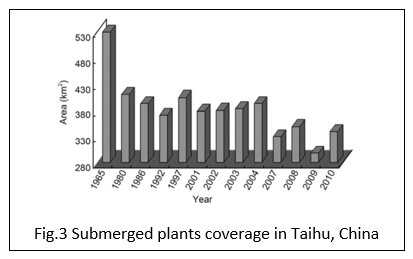 The submerged aquatic plants which are adapted themselves to original lake conditions (e.g. high concentration of chlorophyll) are almost wiped out from the lakebed during the algae bloom (Jupp and Spence, 1977). In the case of Taihu lake in China, the area covered by submerged aquatic plants was over 530 km2 which reduced to around 300 km2 in 2009 (Qin et al., 2012).
The submerged aquatic plants which are adapted themselves to original lake conditions (e.g. high concentration of chlorophyll) are almost wiped out from the lakebed during the algae bloom (Jupp and Spence, 1977). In the case of Taihu lake in China, the area covered by submerged aquatic plants was over 530 km2 which reduced to around 300 km2 in 2009 (Qin et al., 2012).
The decreasing amount aquatic plants would have an impact to the zooplanktons. Less coverage of submerged aquatic plants on lakebed means less refuge capacity provided for zooplanktons (SCHRIVER et al., 1995). Therefore, besides the pressure of hypoxia, zooplanktons are struggling to survive at high predation risk.
One pronounced impact of lake eutrophication is the decreasing trend of overall fish population along with rising algal population as oxygen depleted environment is no longer able to hold big fish population as a healthy lake. Aparting from decreasing quantity of fish community, the fish community quality is also under threat. Generally, highly eutrophic lakes often are dominated by ferocious fish species such as carp (Lee and Jones, 1991). They are more adapted to the poorly oxygenated environment and they are voracious predator of zooplanktons that eat algae, which is an enhancing factor of lake eutrophication (Reinertsen et al., 1990). Decreasing fish community diversity could also happen when low oxygen condition driving deep-water living fish coming to open water under oxygen pressure which result in hybrid with open water fish.

Human interference to the ecosystem
Under such environmental pressure, countries like China decides to apply biotic approach to solve the algae bloom causing by eutrophication. Deploying algae-munching fish is well-known as approach to regulate algae population (Andersson et al., 1978). However, massive releasing algae-munching fish would dramatically changing the composition of current aquatic community leading unpredictable problems in future.
References
Andersson, G., Berggren, H., Cronberg, G. and Gelin, C. (1978). Effects of planktivorous and benthivorous fish on organisms and water chemistry in eutrophic lakes. Hydrobiologia, 59(1), pp.9-15.
Hallegraeff, G. (1993). A review of harmful algal blooms and their apparent global increase*. Phycologia, 32(2), pp.79-99.
Jupp, B. and Spence, D. (1977). Limitations on Macrophytes in a Eutrophic Lake, Loch Leven: I. Effects of Phytoplankton. The Journal of Ecology, 65(1), p.175.
Lee, G. and Jones, A. (1991). Effects of Eutrophication on Fisheries. Reviews in Aquatic Science, [online] 5(3). Available at: http://www.gfredlee.com/Nutrients/Effects_Eutroph_Fisheries.pdf [Accessed 22 Mar. 2017].
McKinnon, J. and Taylor, E. (2012). Biodiversity: Species choked and blended. Nature, 482(7385), pp.313-314.
National Science Foundation, (2000). Estimated Annual Economic Impacts from Harmful Algal Blooms (HABs) in the United States. [online] National Science Foundation. Available at: http://www.whoi.edu/cms/files/Economics_report_18564_23050.pdf [Accessed 22 Mar. 2017].
Qin, B., Gao, G., Zhu, G., Zhang, Y., Song, Y., Tang, X., Xu, H. and Deng, J. (2012). Lake eutrophication and its ecosystem response. Chinese Science Bulletin, 58(9), pp.961-970.
Reinertsen, H., Jensen, A., Koksvik, J., Langeland, A. and Olsen, Y. (1990). Effects of Fish Removal on the Limnetic Ecosystem of a Eutrophic Lake. Canadian Journal of Fisheries and Aquatic Sciences, 47(1), pp.166-173.
SCHRIVER, P., BOGESTRAND, J., JEPPESEN, E. and SoNDERGAARD, M. (1995). Impact of submerged macrophytes on fish-zooplanl phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology, 33(2), pp.255-270.
word count: 496


Recent Comments