Climate change – a myth? We have all heard of it and its impending threat to our global environment. However, what we should ask ourselves is how are plants affected by our planet’s increasing temperatures, carbon dioxide (CO2) levels and the increasing frequency and intensity of severe weather changes?
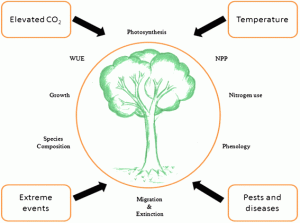
Plants play a critical role in pulling CO2 out of the atmosphere. This uptake of CO2 during photosynthesis is a major pathway by which carbon can be stored (Tkemaladze and Makhashvili, 2016). Carbon dioxide is predicted to increase to approximately 1000 ppm by 2100. Since the beginning of the Industrial Revolution approximately 200 years ago average global temperatures have increased by 0.85°C and by the end of the century temperature is projected to rise by approximately another 4°C (IPCC, 2013). Some would assume this to be beneficial to plants due to these warmer temperatures and increased levels of gas as it should, in theory, encourage growth. However, it is not as straight forward as this.
The enzyme rubisco is the key to this photosynthetic process by fixing CO2. Drake et al. (1997) states that the increased levels of CO2 will allow greater fixation by plants and, therefore, result in increased growth. However, Bisgrove and Hadley (2002) found that long-term exposure to elevated levels of CO2 caused an accumulation of carbohydrates in plant tissues, which in turn reduced the rate of photosynthesis. Furthermore, although plants initially respond positively to increasing temperature, this will eventually plateau or even decline after reaching the optimum range for some species. Plants may experience an increased rate of respiration leading to death; illustrating the world’s plants can easily lose their ability to act as a global carbon sink, becoming instead yet another carbon source (Mellilo et al., 1990; Hawkins et al., 2008).
Moreover, another consequence of global environmental change is a change to global weather patterns. Many do not connect climate change with uncharacteristic weather events, however, there is no doubt that climate change affects their intensity and frequency. Thus, in the future, we can expect to experience more frequent periods of drought, floods and storms (Frich et al., 2002). For example, during the past winter, there was snow escape in Spain as we witnessed a window to our future in the form of the courgette and spinach crisis, which caused havoc and rationing in British supermarkets. Yet these changing weather patterns will have a much larger impact than just a blow to spiralizer sales.

Stated above are only a few effects global climate change has on our planet’s plants. Plants have an essential regulatory role in the control of our planet’s climate: they did yesterday, they do today and they most certainly will in the future. If we continue to allow the CO2 level to increase at the rate it is currently we will suffer dramatic consequences. It not only will affect the Earth’s vegetation such as forests and plants, but will also have a knock-on effect on global food production, therefore, affecting our wellbeing.
[499]
References
Bisgrove, R. and Hadley, P. (2002). Gardening in the global greenhouse: The impacts of future landuse and climate on the red list status of the Proteaceae in the cape floristic region, South Africa. Global Change Biology, 69, pp.79-91.
Drake, B., Gonzàlez-Meler, M. and Long, S. (1997). More efficient plants: A Consequence of Rising Atmospheric CO2?. Annual Review of Plant Physiology and Plant Molecular Biology, 48(1), pp.609-639.
Frich, P., Alexander, L., Della-Marta, P., Gleason, B., Haylock, M., Klein Tank, A. and Peterson, T. (2002). Observed coherent changes in climatic extremes during the second half of the twentieth century. Climate Research, 19, pp.193-212.
Hawkins, B., Sharrock, S. and Havens, K. (2008). Plants and climate change; which future? Richmond, UK: Botanic Gardens Conservation International, pp.98.
IPCC (2013) Climate Change 2013: The Physical Science Basis.Intergovernmental Panel on Climate Change, Cambridge, UK.
Kallarackal, J. and Roby, T. (2012). Responses of trees to elevated carbon dioxide and climate change. Biodiversity and Conservation, 21, pp.1327-1342.
Melillo, J., Callaghan, T., Woodward, F., Salati, E. and Sinha, S. (1990). Effects on Ecosystems, in Climate Change: The IPCC Scientific Assessment, edited by J. Houghton, G. Jenkins, J. Ephraums, Cambridge University Press, Cambridge, pp.283−310.
Tkemaladze, G. and Makhashvili, K. (2016). Climate changes and photosynthesis. Annals of Agrarian Science, 14, pp.119-126.


Recent Comments