The planet needs YOUR help!
We all have been alarmed and warned about rising sea temperatures, melting sea ice and glaciers, but the main problem is that we could describe them on and on.. Therefore, one of the greatest challenges the earth faces in this day and age is CLIMATE CHANGE.

An unprecedented ↑ in atmospheric CO2 and temperature can shockingly lead to:
- Species extinction
- Collapsing ecosystems and food chains and as a result, foreseeable shortages of food and water to the increasing global population.
One of the toughest tasks this century, is predicting the response of plants to global climate change. Thus, what is essentially happening to our plants through this unpredictable change?
Many studies have observed:
Plants will thrive with an established elevation in CO2 and temperature…
- As more CO2 = more fixation = more photosynthesis
Bisgrove and Hadley (2002) suggest that a “doubling in carbon dioxide level can increase plant growth by as much as 50%”
- More heat = advanced growth and seed germination
HOWEVER…
Agriculture sustains almost all life-forms on Earth, meaning adverse conditions, can restrict crop plants in reaching their full genetic potential of producing a high yield (Anjum et al., 2014).
(1) Heat waves, extreme temperature events are projected to become more intense, more frequent and longer lasting to what is currently been observed in recent years (Hatfield and Prueger, 2015). Consequently, when a drought occurs where the levels of heat are extreme, the growth of a plant will rapidly decrease due to the high level of moisture loss. Furthermore, although water is essential for the functioning of virtually every plant on the planet, too much water (as a result of a storm… which we all can undeniably find exciting!) can in fact reduce the amount of oxygen in the soil, making a plant more susceptible to disease.
NOTE: Remember to stop and think, ‘this storm is not only damaging our plants, but in fact our livelihoods!’
(2) In addition, there is abundant evidence that in the long term, plants will begin to acclimate to a rise in CO2 levels. Therefore, the photosynthetic capacity becomes inhibited due to the plants being unable to utilise the additional carbohydrate that is accompanied with photosynthesis (Drake et al., 1997).
(3) Shockingly, crops of the future that are grown in a high- CO2 environment, will have decreases in the concentrations of zinc, iron and protein in grains of wheat, barley and rice (Myers et al., 2014) meaning the food in which we eat will be much less nutritious. Considering most people depend on these grains for their source of zinc and iron this can be detrimental to human health.
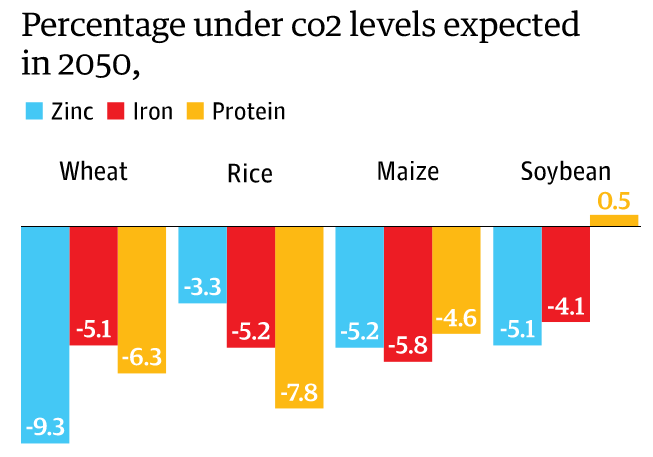
If we continue to actively contribute to a world of increasing CO2 and temperature, not only are the plants on our Earth disturbed but if you stop and think: YOU yourself can be hugely affected.
A question of thought, who knows what this may cause to our future health and livelihoods?
References:
- Anjum, N., Gill, S. and Gill, R. (2014). Plant adaptation to environmental change. 1st ed. Wallingford: CABI.
- Bisgrove, R. and Hadley, P. (2002) Gardening in the Global Greenhouse: The Impacts of Climate Change on Gardens in the UK. Technical Report. Oxford: UKCIP.
- Drake, B., Gonzalez-Meler, M. and Long, S. (1997). MORE EFFICIENT PLANTS: A Consequence of Rising Atmospheric CO2?. Annual Review of Plant Physiology and Plant Molecular Biology. 48(1), pp.609-639.
- Hatfield, J. and Prueger, J. (2015). Temperature extremes: Effect on plant growth and development. Weather and Climate Extremes, 10, pp.4-10.
- Myers, S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, A., Bloom, A., Carlisle, E., Dietterich, L., Fitzgerald, G., Hasegawa, T., Holbrook, N., Nelson, R., Ottman, M., Raboy, V., Sakai, H., Sartor, K., Schwartz, J., Seneweera S., Tausz, M. and Usui, Y. (2014). Increasing CO2 threatens human nutrition. Nature, 510(7503), pp.139-142.
Word Count: 492






Recent Comments